COVID-19 Face Shield PPE
Version last updated 4/2/2020 @ 1:00pm Pacific
Supplemental Information
for DtM-v3.0 Face Shield
Graphical Instructions for Use
Product Point of View
Healthcare workers responding to COVID-19 who face PPE (personal protection equipment) supply gaps while waiting for domestic face shield production to catch up with demand NEED a transparent face shield that:
limits aerosol and splatter exposure from in front and above, while providing top ventilation
reduces aerosol and splatter exposure on N95 and other face masks
is re-usable for a single user (can survive multiple daily washes; transparent visor can be replaced from readily sourced materials when worn out)
is easy to fabricate within a few days of design approval (ie no complex supply chains or production bottlenecks)
is comfortable to wear and easy to don and doff (as it will be taken on and off dozens of times in a twelve-hour shift)
provides protection to broader area of face compared to standard safety goggles or glasses
One ER nurse on the front lines at a Seattle-area hospital, wearing the DtM-v3.0 face shield for the first time on Friday March 27 2020, said:
"I love it. It makes me feel safer. I was swabbing someone for covid-19 when he vomited on me. It kept me clean."
Bill of Materials
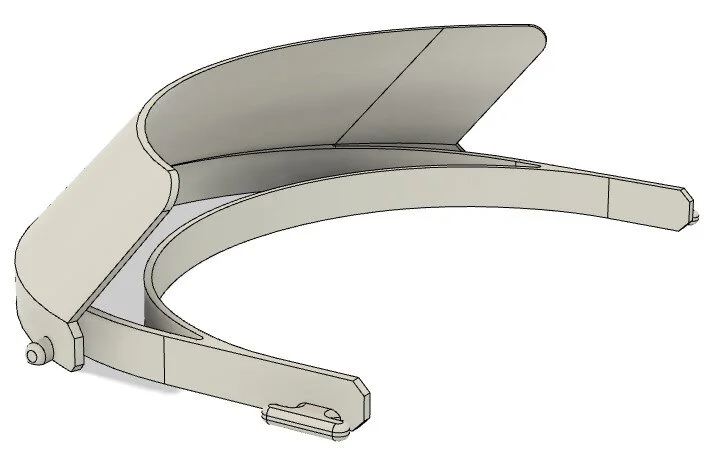
3d-printed headband in PLA, roughly 50g/1.75oz per part. Outer envelope of 3d-printed headband is 191mm wide, 148.5mm long, and 52.5mm tall. Print time is roughly 3h15m per part on a Prusa i3 MK2S and a Lulzbot Taz 6 at 30% infill, no supports.
Acceptable alternate 3d printing materials include PETG, ABS, ASA, Nylon
Elastic for the headband: could be 7”x1/8” rubber bands, 13” strip of 3/4" wide buttonhole elastic, coflex/coban tape
A standard US letter-sized transparency or report-cover for the shield, 2-10mil (0.002-0.01”, 0.05-0.25mm) thickness
Acceptable alternative materials include clear PETG, PMMA or mylar in the same thicknesses cut to 8.5” x 11” (215.9mm x 279.4mm). Dimensions for a US-style three-hole-binder punch: three 6-8mm diameter holes with each center spaced 108mm apart. Do NOT cut PVC
Options:
Clinicians and caregivers who have worn the device on service recommend the following additions to the headband to improve comfort: add a wrap of foam tape or "chest tube foam tape", tape layers of gauze or a folded paper towel on the headband; dispose as necessary
Before hole-punching, add tape (duct tape, medical cloth tape, etc) to reinforce the holes at top of the transparency sheet during repeat use and washings. Remove and replace tape between patients as necessary.
Instructions for Use and Assembly
Punch holes in standard US letter-sized transparency (8.5x11 in) with a standard US three-hole-punch.
Attach transparency to headband on the three mounting pegs.
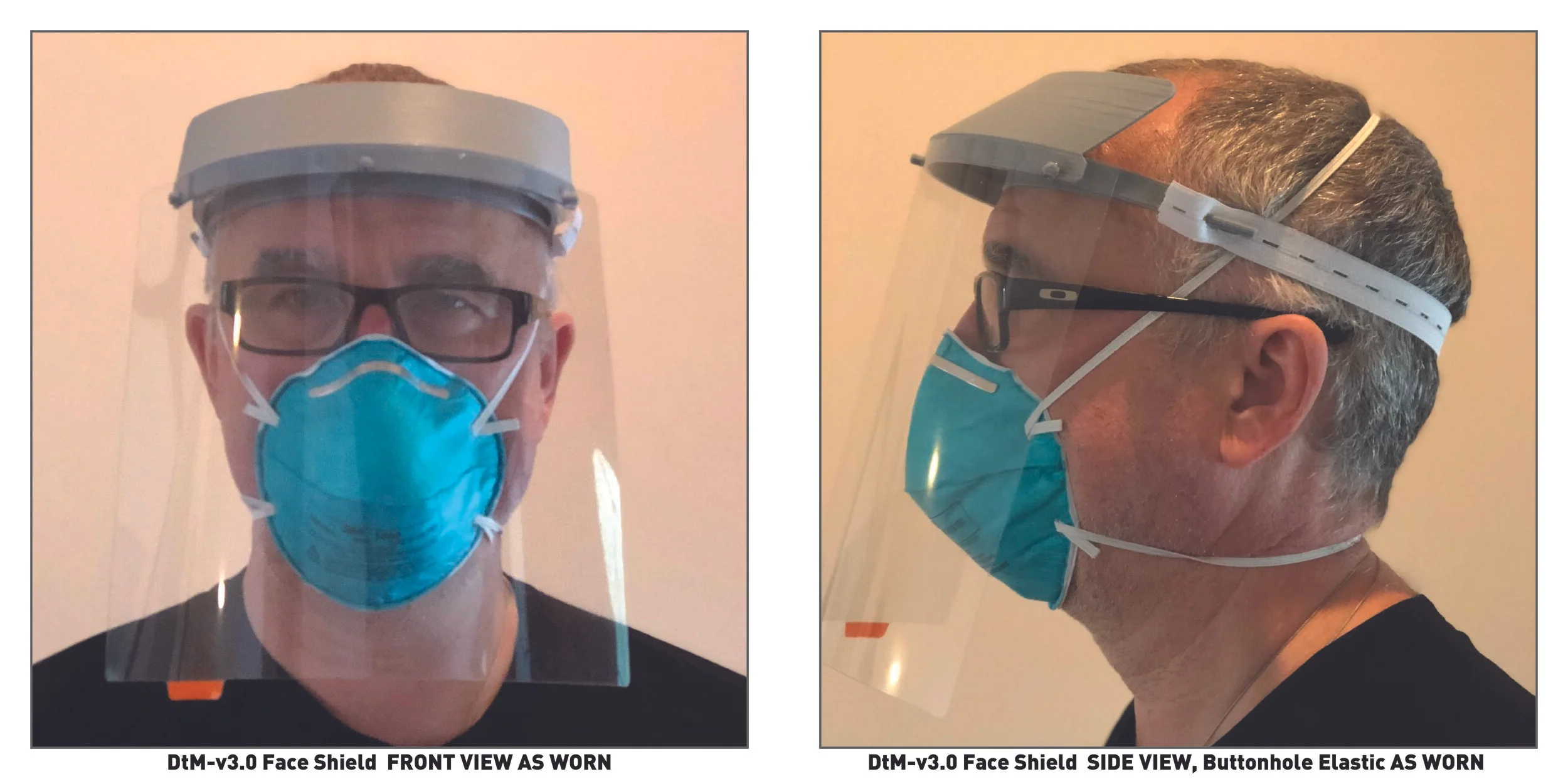
Attach elastic to headband with cleats near temples, adjust to fit. Some users may find 7” rubber band too tight, consider chaining elastic bands as necessary.
To clean, follow CDC recommendations in Strategies for Optimizing the Supply of Eye Protection - Selected Options for Reprocessing Eye Protection. DO NOT submerge or soak 3D-printed headband in cleaning solution as the headband may absorb the solution and leak it out onto the wearer’s forehead over time.
Discard and replace the transparent visor as appropriate, after excessive wear or fogging.
DtM-v3.0 Face Shield CAD VIEW
DtM-v3.0 Face Shield DESIGN REFERENCE VIEWS
DtM-v3.0 Face Shield DESIGN REFERENCE VIEWS, AS WORN
DtM-v3.0 Face Shield compatible with replacement headband made from coban-LF, common medical consumable.
This design is a remix from the Prusa Protective Face Shield - RC2. We are grateful to the team at Prusa for their design skills, their commitment to open hardware, and their leadership in a crisis.
Team
We extend a big thank you to our collaborators
Design Team Volunteers
Elizabeth Johansen - Spark Health Design
David Packman, Microsoft
Eric Moyer, Boeing
University of Washington Harborview Medical Center
Vanessa Makarewicz, MN, RN, CIC
Dr. Graham Nichol
Glenn Allen, Operations Manager
Gwen Angel, BSN
University of Washington Medical Center
Dr. Dmitry Levin
Dr. Paul Pottinger
Danica A Little, MHA
Dr. David Hananel
Dr. Ross Kessler
Mass General Brigham
Dr. Kris Olson, Pediatrician, Internist, MGH
Dr. Paul Currier, Pulmonologist, MGH
Outreach Broadcast
Malory Johnson, ELEVEN
Microsoft Garage Volunteers
Jeff Ramos
Linda Thackeray
Stacey Mulcahy
Steve Scallen
Chris Templeman
Duke University
Tommy Sowers
Saige Sunier
Eric S Richardson
Olin College
Benjamin Linder
UWMC Supply
Josh Bakelaar
Cherry G Pangilinan
Suzanne Heikka
Katie C Friday
UW Engineering Fabrication
Colleen Carroll
Jeffrey I. Lipton
Prof. Pierre D Mourad
Jason R Speich
DJ Traina
Imen Hannachi
MSF Tokyo
Michiko Kyokan, RN
Stefano Di Carlo
Massimo Ravasini
Maker Community Volunteers
Jeremy Hanson, Seattle Makers
Tim Butterworth, Artisans Asylum
Joel Joseph, USC
Washington Open Fabricators
Tom McNulty, Autodesk
Chris Templeman
Shehryar Siddiqui, CAPM
Mike Eisenstein, Path
Victory L, Stop the Bug
Tomeo Wise, Studio Fathom
Volunteer Connectors
Elizabeth Bruce, Microsoft
Kent Foster, Microsoft
Stephanie Howard, How and Why Design
Ben Beck, ELEVEN
Elizabeth Cross Nichol, Downstream Therapeutics
In addition, DtM thanks the many others who took the time to provide feedback on the design from the University of Washington Medical Center, MGH, Global Good, and the Open Source COVID-19 Medical Supply group communities.